Designing a Hand Warmer Lab Report November 26th 2017 Period 4 I. With the Designing a Hand Warmer Advanced Inquiry Lab Kit for AP Chemistry students investigate energy changes and calorimetry with formations of solutions.

Designing A Hand Warmer Lab Sheet Docx Designing A Hand Warmer Lab Ap Chemistry Problem The Purpose Of This Advanced Inquiry Lab Is To Design An Course Hero
The vessel in use will be a coffee cup calorimeter where six different solids will be dissolved in.

. To determine the best solute to use to make a safe hand warmer. When chromium chloride CrCl 2 is dissolved in water the temperature of the water decreases. Cover the cup and record the temperature using a thermoprobe.
Sandra Bergh Created Date. Up to 24 cash back use and inexpensive hand warmer. Of a substance to make your own hand warmers.
The heat of reaction ΔHsoln is written after the products in units of kJmol rxn. 1 Place an opened Ziploc bag into a beaker and fold the edges over the side of the. 4Fe s 3O 2 g - 2Fe 2 O 3 s In todays lab we will explore how the rusting process is used in hand warmers.
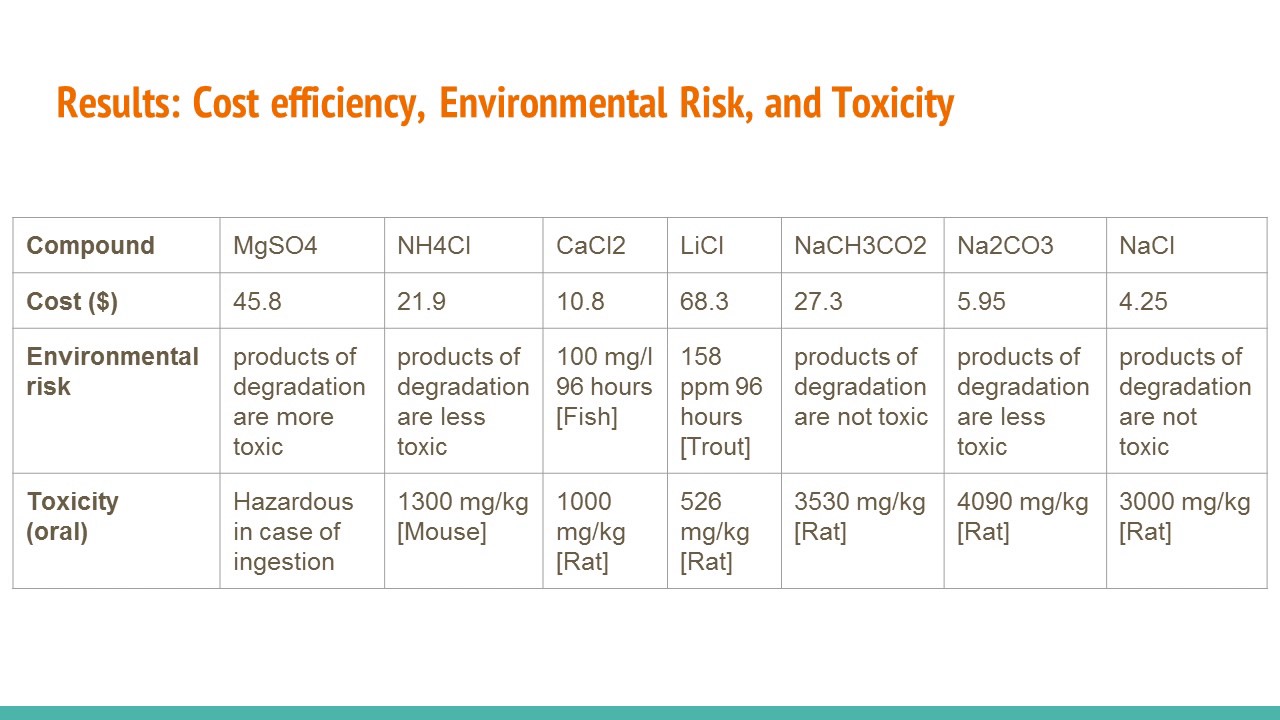
During the lab six different solids three different chemicals for each group along with their costs and individual Safety Data Sheets. The rusting is a redox reaction and the equation is as follows. We calculated the different amount of heat that was.
For example sodium hydroxide dissolves exothermically releasing 442 kilojoules per mole dissolved. Microsoft Word - Grading Rubric-Designing Hand Warmer Labdocx Author. Making a reusable hand warmer warm super saturated sodium acetate solution beaker e filter funnel filter paper filtrate 3.
Term papers 8 pages physics published on 7 July 2011. Hand warmers work because of a rusting process. Create a hand warmer using a substance that is safe cheap and emits the most heat.
You will carry out an experiment to determine which substances in what amounts to use in order to make a hand warmer that meets these criteria. Designing an Economical Hand Warmer Purpose The purpose of this investigation is to investigate energy transfer using calorimetry and ultimately design the most cost effective handwarmer possible from a given set of chemicals. This document was updated on the 12072011.
The chemical that will likely be the best for use in a hand warmer is the sodium carbonate because it is the least toxic of the three chemicals while still producing an exothermic reaction. Heat of Solution measures. There are several types of hand warmers commercially available that take advantage of different chemical reactions to provide a gentle heat for an extended period of time.
Breaking bonds and particulate. From instant cold packs to flameless ration heaters and hand warmers the energy changes accompanying physical and chemical transformations have many consumer applications. Heat of solution for an ionic compound.
And safety information to propose a design for the best all-around hand warmer. Up to 24 cash back Conclusion - Hand warmer challenge. See more product details.
Measure heat transfer using a calorimetry investigate energy changes accompanying the formation of substances and design a hand warmer that is reliable safe and inexpensive. A very cheap and effective hot pack design was made from a Ziploc bag and duct tape which insulates the pack and helps keep it warm. Up to 24 cash back Designing a Hand Warmer Lab Introduction.
Give all your data and do step 3 calculate the molar heat of solution for each. The backbone of these applications is. Students challenge themselves to design the best all-around hand warmer.
NaOH s -- Na aq OH- aq ΔHsoln -442 kJmol rxn Write equations to similarly represent the dissolving process for. The process of kinetic energy transfer at the particulate scale is referred to in this course as heat transfer and the spontaneous direction of the transfer is always from a. 1 Measure out 2 separate samples of 1000 mL of distilled water.
One example is the hand warmer. Which is strongerthe attractive forces between water molecules and chromium and chloride ions or the combined. Designing a Hand Warmer Purpose.
This lab will require you to put your chemistry skills to commercial use. Q aq Mass x Specific Heat x Temp Change q cal Temp Change x C cal q soln - q aq q cal. One type of hand warmer contains a mixture of dry chemicals including among other things powdered iron and salt.
Substances are lithium chloride calcium chloride and sodium carbonate. Pour 5 grams of the substance and 45 mL of water into a cup. Up to 24 cash back Title.
Is the heat of solution exothermic or endothermic. Jonathan Contreras Zane C Natalia R Mercy 2720 Hand Warmer Lab Report Purpose The purpose of this lab is to design the most effective reliable hand warmer at the cheapest price by analyzing different chemical reactions through calorimetry the measure of the transfer of heat. 2 Heat one to about 50C and place other one in calorimeter at around 20C 3 Add heater water to calorimeter cover top wait 15 seconds measure temp.
This process is represented as. Designing a Hand WarmerAdvanced Inquiry Laboratory Kit. Background Hand warmers are a common site in cold weather conditions.
In the experiment we used three types of Chemicals. Of some chemical reactions. 13- calculate calorimeter constant Part B and C.
Include Designing a Hand Warmer Thursday November 17 2016 1028 AM Lab Reports Page 1. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. CaCl2 Calcium Chloride MgSO4 Magnesium Sulfate and LiCl Lithium Chloride.
Discussing the criteria for the hand warmer design and why you are choosing one over the other. Place a hair snapper into the clear plastic bag. Table Part B and C.
7 Calculate the molar heat of solution q solution -q aq q cal -m s ΔT C cal Δ T. Term papers 8 pages physics published on 7 July 2011. Hannah Boster Seth Lewis and William Makinens recorded powerpoint presentation summarizing our work on the Flinn Handwarmer Lab.
Up to 24 cash back Lab 12. Filter your super saturated sodium acetate solution to remove any last remaining solid. Purpose The Purpose of this advanced inquiry lab is to design an effective hand warmer that is inexpensive nontoxic and safe for the environment.
Looking at the results we saw which chemicals created more amount of heat when its being applied to water and salt. Energyenthalpy change with process of dissolving a solute in solvent. In this lab you are challenged to use chemistry to design an effective safe environmentally benign and inexpensive hand warmer.
Your lab group did 3 different compounds.

Designing A Hand Warmer By Makayla Sabo
Designing A Hand Warmer Lab Youtube

Designing A Hand Warmer Lab Alyssa Andrade Ms Sherman Ap Chemistry October 25 2016 I Title Designing A Hand Warmer Ap Chemistry Big Idea 5 Big Course Hero

Designing A Hand Warmer Lab Sheet Docx Designing A Hand Warmer Lab Ap Chemistry Problem The Purpose Of This Advanced Inquiry Lab Is To Design An Course Hero

Designing A Hand Warmer Lab Alyssa Andrade Ms Sherman Ap Chemistry October 25 2016 I Title Designing A Hand Warmer Ap Chemistry Big Idea 5 Big Course Hero

Designing A Hand Warmer Designing A Hand Warmer Purpose Of Experiment Research And Design An Studocu
0 comments
Post a Comment